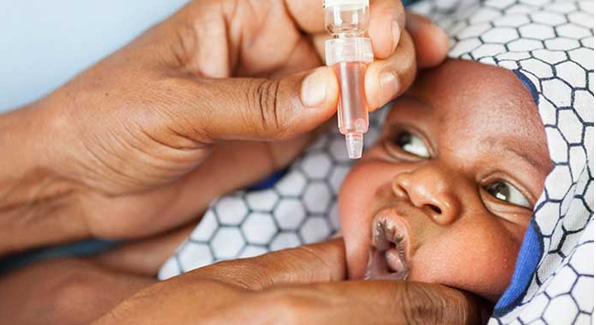
A trial of the vaccine, known as BRV-PV, has suggested it is both safe and effective in protecting children against rotavirus – the leading cause of severe diarrhoea, which is the second biggest killer of infants and children around the world.
Rotavirus itself is linked to 1,300 deaths per day, primarily in sub-Saharan Africa. The vaccine has been adapted to the two most common strains of rotavirus found in the region’s countries.
BRV-PV is different in another way, however. The two vaccines currently available can’t withstand the heat of the continent, and require refrigeration at all times.
This makes BRV-PV a “game changer”, Michaela Serafini, medical director at NGO Médicins Sans Frontières, said.
Most deaths due to rotavirus occur in low-income nations, where access to clean water and sanitation is limited, and especially in rural areas. In such situations, vaccines can have an enormous impact, but only if they can survive the journey to the people they need to reach.
Without the need to stay cool, BRV-PV will be much easier to transport to the remote communities that need it. Procedures for transporting current vaccines are also unreliable, meaning vaccines can perish en route.
As well as saving on transportation complexity and cost, BRV-PV is far more affordable. A full-price dose of BRV-PV costs just $2.50 – the same as the subsidised price of the cheapest other vaccine for rotavirus currently available.
BRV-PV is currently awaiting approval from the World Health Organization, after which it could also become available at a subsidised price, making it significantly cheaper than other vaccines on the market.
The study, published today in the New England Journal of Medicine, examined the efficacy of BRV-PV in Niger. The trial involved more than 4,000 children under the age of two. Some were given a full course of the vaccine (three doses), others received at least one dose, and others received a full or short course of placebo.
It provided evidence that BRV-PV is not only safe, but effective. Results showed a 66.7% efficacy among the group that received the full course of the BRV-PV.
“The success of this trial shows that research and development into vaccines that are specifically adapted for use in low-income countries yield results,” said Serafini.
“The quicker this vaccine is prequalified by the WHO, the sooner it can be used to prevent the deaths of thousands of children in the countries where it is most needed.”